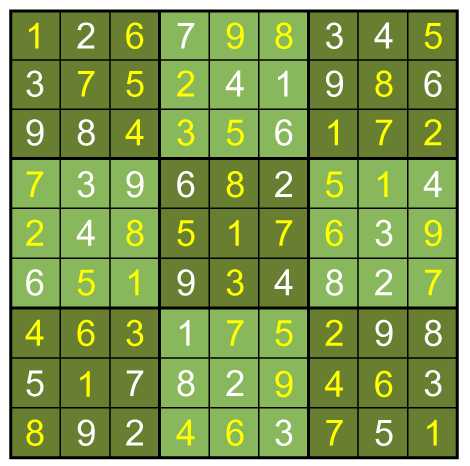
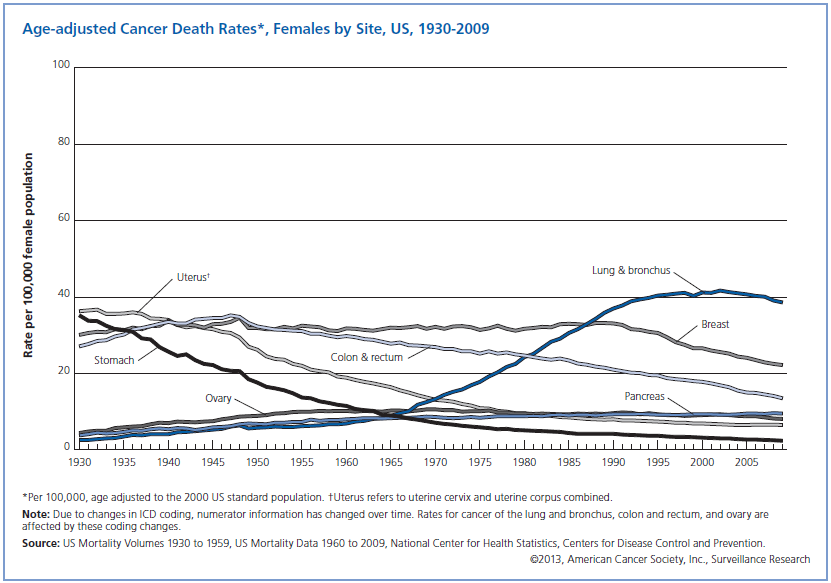
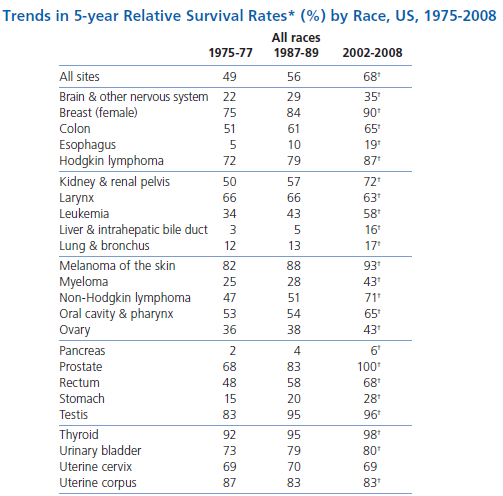
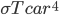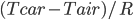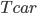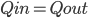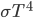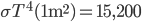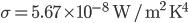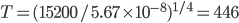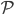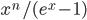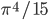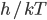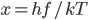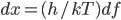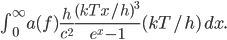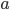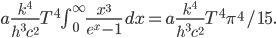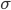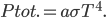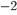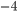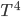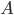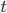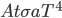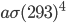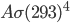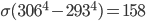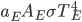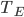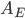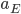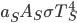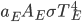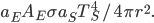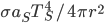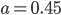It's possible to solve a Gentle Sudoku with only a box-by-box search technique. While this is the first technique I will normally apply, it is not the only one necessary to solve most Gentle puzzles.
Sudoku has 3 rules: every 3x3 highlighted box must have 1-9, every row must have 1-9, and every column must have 1-9. In a box-by-box search, you use the row and column rules to solve squares in a given box. As you would guess, we can also do a row-by-row search that uses the box and column rule to solve squares.
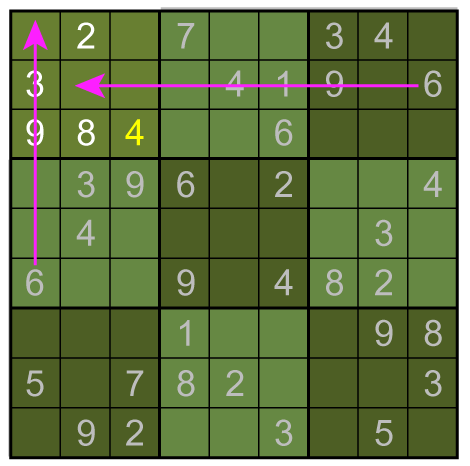
Consider:
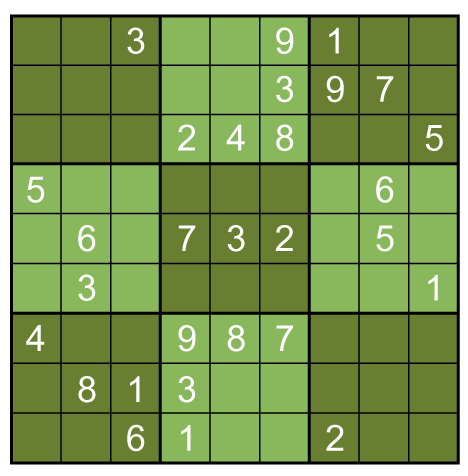
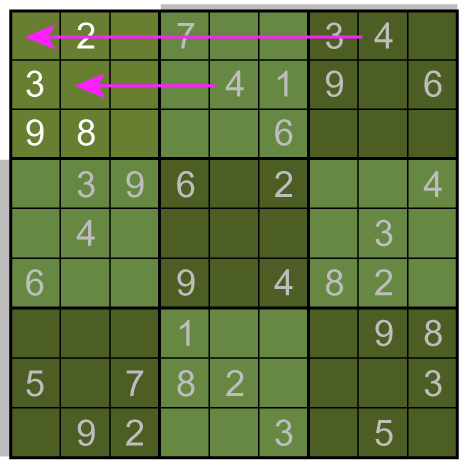
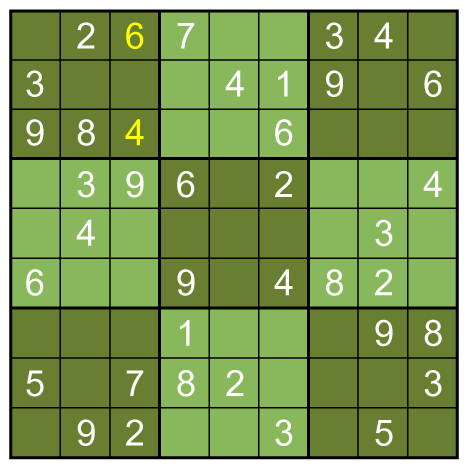
A box-by-box search pattern allows the solution for 12 squares, indicated in yellow:
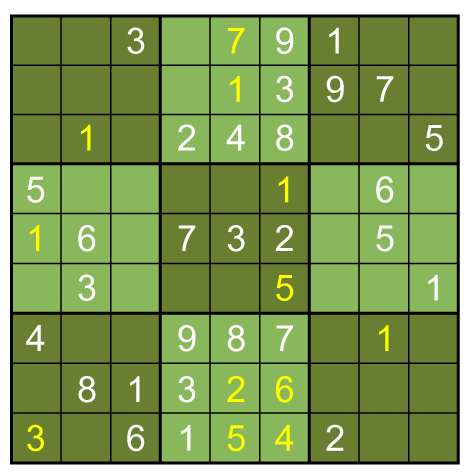
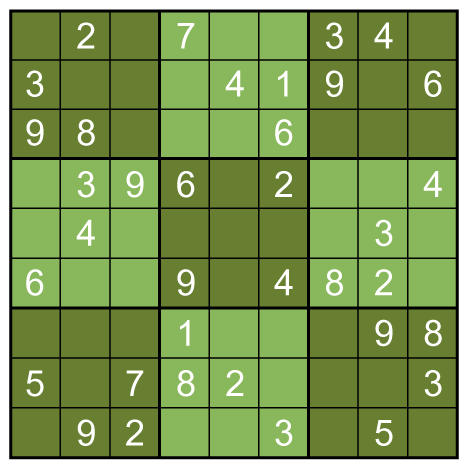
No more such inferences can be made at this point. However, the puzzle is vulnerable to a row-by-row search. Notice that the 8th row can only have a 5 in its seventh space, due to the other squares being excluded by the 5s above them: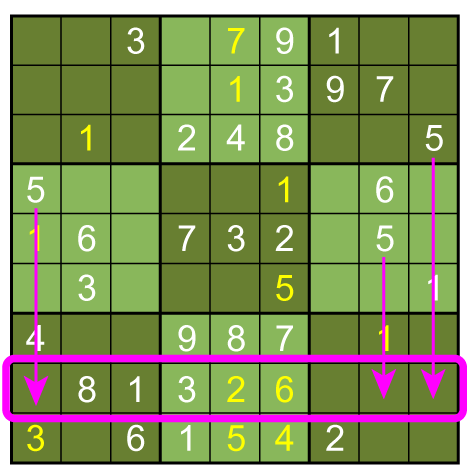
Thus, the 5 must go in box 9, square 4.
Technique 2: Row by row
Search pattern: go through each row and check every number that doesn't already appear in the row. Exclude squares from the row that would violate the column or box rule.
Logically, the final basic technique is a column by column search, exactly analogous to the row-by-row method.
Technique 3: Column by column
Search pattern: go through each column and check every number that doesn't already appear in the row. Exclude squares from the row that would violate the row or box rule.
In this case, the fifth column must have its 6 in sixth box: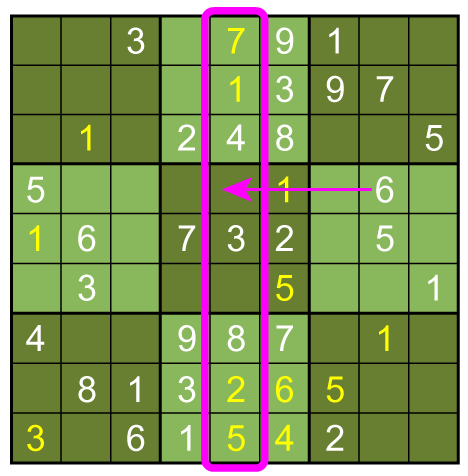 A combination of the three basic techniques leads to the solution below.
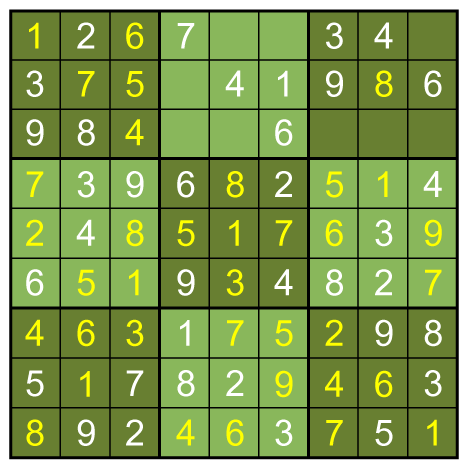
A combination of the three basic techniques leads to the solution below.
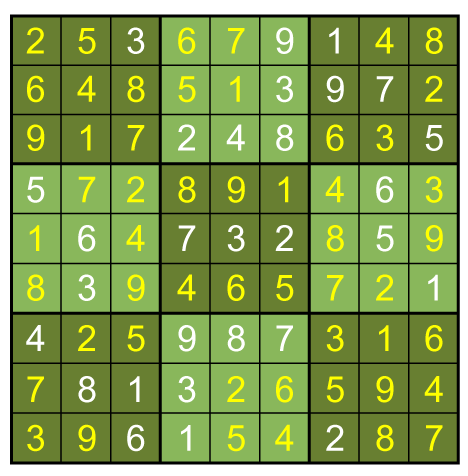
To get there I used 3 more column-by-column solves (box 5 square 2=9, box 3 square 8=3, box 7square 8=9), then nine more row-by-row inferences. The remaining square can be filled in with a box-by-box search or by following the direct Sudoku rules (filling the remaining square in a group with the only entry that isn't there).
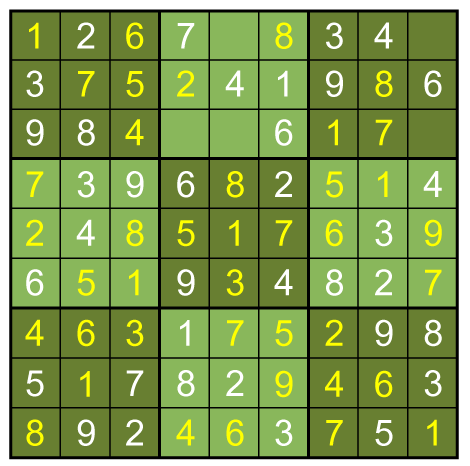
Full solution:
(note: Boxes are numbered left to right, top to bottom like words on a page. Squares are numbered the same way within a box)
box-by-box:
2,5=1
4,4=1
1,8=1
2,2=7
5,3=1
Therefore 5,9=5
7,7=3
8,5=2
8,6=6
8,8=5
Therefore 8,9=4
9,2=1
row by row
9,4=5
col by col
5,8=6
Therefore 5,2=9
3,8=3
7,8=9
row by row
9,9=7
Therefore 9,8=8
7,4=7 (also possible box-by-box)
1,9=7
1,7=9
Therefore 3,7=6
9,3=6
9,1=3
6,3=3
Box-by-box
4,2=7
6,7=7
6,8=2
6,6=9
9,5=9
Therefore 9,6=4
Therefore 3,2=4
4,3=2
4,9=9
4,6=4
Therefore 4,7=8
Therefore 5,7=4
Therefore 5,1=8
Therefore 6,1=4
Therefore 6,4=8
7,2=2
Therefore 7,3=5
Therefore 1,6=8
3,3=8
Therefore 3,6=2
1,1=2
Therefore 1,4=6
1,5=4
Therefore 1,2=5
Therefore 2,1=6
Therefore 1,4=5