All cars have to keep themselves cool. The reason for this, as we've seen, is the inefficiency of the engine: you can't convert the heat, made in the burning of gasoline, into work without creating some waste heat. If we take the energy stored in gasoline to be the "free energy" of the chemical bonds, then a car engine is about 18% efficient, which means that 18% of the heat created is turned into work by the engine. The other 82% of the heat has to be thrown off by the car somehow, or the temperature would just keep rising, which we know doesn't happen (the car would overheat, the oil would burn, etc. Bad things.) What is the technological method for getting rid of all this excess heat?
Quite obviously, the only place for the car to shed its heat is into the atmosphere. It does so by several methods. First, some (a lot) of the heat is in the exhaust gas emitted from the tailpipe. Second, there is a big fan that blows air into the engine from the front, so that the air carries away heat by convection. The car also conducts heat directly into air in contact with it, and because of convection this will be substantial. Finally, we have radiation. A motor circulates water through the engine and through something specifically designed to radiate heat well. It's called a, guess what, radiator. The radiator sends the heat out as IR photons that are absorbed by the hood of the car. The hood then either radiates that heat out into the world or conducts it to the world.
When the car first starts running, it is not capable of shedding all the heat. This is because the temperature is low, and we know that the rate of heat radiation per area is 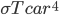 , and the rate of conduction is
, and the rate of conduction is 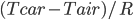 . When
. When 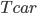 is low, these rates are low. But as the parts heat up, they can radiate and conduct better and better. The stable temperature is when the heat produced in the engine is equal to the heat flowing out through the body of the car by any means. In other words, steady state is when
is low, these rates are low. But as the parts heat up, they can radiate and conduct better and better. The stable temperature is when the heat produced in the engine is equal to the heat flowing out through the body of the car by any means. In other words, steady state is when 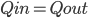 .
.
Let's see if we can estimate how hot the hood of a car gets, assuming the car throws off most of its heat just by radiation. When the car is going 65 miles an hour, it needs about 25 horsepower, which is about 18,600 watts (that's 18600 joules every second). 82% of that is waste, or about 15,200 watts. This is the amount of heat the car needs to get rid of when it reaches a stable temperature. The radiation rate per area is 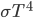 , times the emissivity which we will assume is 1. We need the area of a hood, which is about 1 square meter. So
, times the emissivity which we will assume is 1. We need the area of a hood, which is about 1 square meter. So 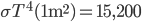 watts. With
watts. With 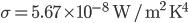 , this becomes
, this becomes 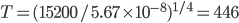 celcius!!! That cannot be right! Car hoods are nowhere near that hot.
celcius!!! That cannot be right! Car hoods are nowhere near that hot.
There's nothing wrong with the physics, we just made a bad assumption (that most of the heat leaves by radiation). This proves a point: thermodynamics in a real system like a car is hard to analyze. We have to start thinking about other ways that heat could be lost. The most obvious way is through exhaust. Exhaust gases are about 530 Kelvin. So the heat loss from that is the volume of gas, times its heat capacity per volume, times the temperature, and that's liable to be pretty high, especially given the relatively high heat capacity of water vapor. The car is also radiating downward from the undercarriage to the street. Finally, there is air flow over the car, carrying away a significant amount of heat from convection.
Human beings are even more complicated. Do humans always need to lose heat? Yes, they do. People are constantly running various machines in our body, and all of that machinery creates waste heat. In fact, your normal metabolism creates about 90 watts (90 J of heat every second), the same as a pretty bright light bulb, and that's when your body isn't even moving. If you couldn't shed that heat, body temperature would rise, and that would spell death before too long. But how does it shed the heat? Here are some ways:
- heat conduction through your clothes to the air or through your skin directly into the air.
- radiation by your skin or clothes
- evaporative cooling from sweat
- energy stored in your outgoing breath
- convection (especially if there's air flow)
Unlike a car, we don't exhaust that much heat through breathing. Most of our heat loss is due to radiation.
The temperature you feel like is dependent on whether the amount of heat you're shedding per second is above or below 90 W. But humans respond to the changes in heat loss to counteract them. They sweat, which carries heat away by evaporative cooling, but how much heat depends on the relative humidity and the rate of air flow
(a good pic of this is here).
Now suppose you move around a bit. Your metabolic rate increases due to extra chemical processes, or extra engine cycles. And the airflow is different because you're moving through the air, so the effect of evaporative cooling is enhanced, but only if it's dry outside. And if you're even receiving indirect sunlight, then the radiation is larger. Very complicated.
If you're standing in a room at temperature  , there is heat conduction through your clothing or from out of your bare skin. This happens until the layer of air directly around you reaches the skin temperature, whereupon it stops. In a very still room this happens fairly quickly. If any of the air is moving, though, the air around you will be replaced with room temperature air and heat conduction resumes. If there is steady flow, then you have constant heat flow from your skin. If it's relatively cool, a breeze will carry a lot of heat away from you. But the temperature in the room is the same whether or not there's a breeze! A thermometer will register no difference between a breezy cool day and a still cool day. However, of course you feel cooler with the breeze.
, there is heat conduction through your clothing or from out of your bare skin. This happens until the layer of air directly around you reaches the skin temperature, whereupon it stops. In a very still room this happens fairly quickly. If any of the air is moving, though, the air around you will be replaced with room temperature air and heat conduction resumes. If there is steady flow, then you have constant heat flow from your skin. If it's relatively cool, a breeze will carry a lot of heat away from you. But the temperature in the room is the same whether or not there's a breeze! A thermometer will register no difference between a breezy cool day and a still cool day. However, of course you feel cooler with the breeze.
People don't feel the temperature of things we come into contact with, but rather we feel the temperature of our skin. If your skin is colder than about 93 F, it is losing heat at a rate faster than normal, and you feel cold, and if it's gaining heat so that skin temp rises above 93 F, if feels hot. So, if you're walking on red hot (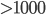 C) coals, your skin never says "1000C!!!", it only states how much the skin has risen in temperature. Walk quickly enough, and minimize the time your foot is in contact with the coals, and your feet stay at a low temperature, because there hadn't been enough time for significant heat to transfer. Walk slowly, and you can expect to be nursing a nasty burn.
C) coals, your skin never says "1000C!!!", it only states how much the skin has risen in temperature. Walk quickly enough, and minimize the time your foot is in contact with the coals, and your feet stay at a low temperature, because there hadn't been enough time for significant heat to transfer. Walk slowly, and you can expect to be nursing a nasty burn.
Even the way your body feels in a still room is affected by multiple factors. Suppose you set your thermostat to 77 F all year. When you get home in the evening during the winter, your walls have a lower temperature than the air in the room. Therefore the radiation from the walls is relatively low compared to other times. In the summer, your walls are warmer than the air, and so they radiate more. The conduction is the same for both, since your body is the same temperature and so is the room. But the actual feeling depends also on the radiation. So people feel colder in the winter, even though the air is the same temperature inside, because there is less radiation hitting them from the outside world than during the summer.
Now, as complicated as all of this sounds, thinking about the thermodynamics of the whole Earth isn't quite so bad. This is because the atmosphere has NO conduction or convection with anything else. The temperature of Earth is totally determined by radiation. The sun shines a certain energy and the earth re-radiates the same amount. If that didn't happen, our planet would be uninhabitable in a hurry.





