At last we know all 3 laws of thermodynamics: Internal energy of a system increases when heat is added to it and decreases when it does work (). No cyclic process can turn heat entirely into work. Absolute zero cannot be attained. The first two of these statements encompass all of what we think of …
Category Archive: Science
Oct 05 2012
Understanding polls (part 1)
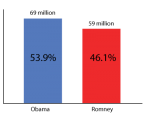
Let's say that we want to know how many people are going to vote for Obama and how many for Romney. The population of all voting people has a certain distribution (a number who vote for one, another number who vote for the other). For the sake of argument let's say it looks like this: …
Sep 17 2012
Thermo for Normals (part 23): Absolute Entropy and the Third Law of Thermodynamics
Here I've been blabbering on and on about entropy, but all I've really talked about is changes in entropy. Irreversible things miss opportunities to do work, and that makes entropy go up. But what is the entropy of, for instance, a bottle of gas? It has pressure, temperature, volume, and internal energy. If the gas is …
Aug 27 2012
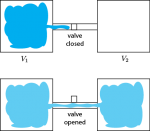
Thermo for Normals (part 22): Other ways to create entropy
I have to admit, I've been fibbing a bit. It is absolutely true that changing something's temperature by heating changes its entropy by . However, this is not the only way to change it. In fact, anything that's irreversible increases the entropy. And there are ways to decrease the entropy of a system without taking …
Aug 06 2012
Thermo for Normals (part 21): Time reversal and breaking the 2nd law
Suppose you are recording a video of someone playing billiards. The balls crash into each other, and if you sit down and calculate the energy and momentum of the balls before and after a collision you will find that energy is conserved and momentum is conserved in each collision. Now, if you reverse the video, …