At last we know all 3 laws of thermodynamics:
- Internal energy of a system increases when heat is added to it and decreases when it does work (
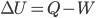 ).
). - No cyclic process can turn heat entirely into work.
- Absolute zero cannot be attained.
The first two of these statements encompass all of what we think of as classical thermodynamics, and they actually describe a ton of stuff without ever needing to think about the atoms, what the forces between the atoms are, or quantum mechanics. You can start to understand engines, air conditioning, evaporation, condensation, and all sorts of other things that you see all the time with just these few rules.
The laws are actually quite general in my opinion. This is a double-edged sword: they are widely applicable, but when you run into a problem, the laws aren't specific enough to resolve them. An example comes to mind. If you put salt into water, the boiling point of the solution is higher than the boiling point of water, due to the extra mass. It happens because you added small particles to the water. What the hell is "small" anyway, though? What if I put a big rock into the water...would it still have this effect?! This is the limit of thermodynamics. Since we aren't really talking about the things that actually exist, we are not able to resolve issues like this.
This doesn't mean that it's useless by any means. In fact, just what we've touched on so far is extremely useful and interesting. But if we want to know more specifics, we can't ignore the actual atoms, their actual velocities, and such. That's where we're heading, and that's where we can really get to know what entropy and internal energy really are. However, it requires use of statistics and probability, and also a knowledge of logarithms and exponential functions. If the reader was to tune out now, I would understand.
But I think that in continuing on, a little bit of effort in thinking about these concepts yields great reward. I've said that in a gas some of the atoms are going faster than others. But you might wonder: well, how many are moving fast? How much faster are they going? To answer these and other questions, we have to change from thermodynamics to statistical mechanics (really just a fancy way of saying that you want to generalize about the laws of mechanics to large numbers of particles). It will turn out in the end that this topic has a few easy to remember rules; we just need to do a little bit of work to get to those rules.





