Every object, including you, radiates all the time. The radiation comes in the form of light, also known as electromagnetic radiation. Most of the time, this light isn't visible. Right now I'm radiating, and almost all of what is coming off is infra-red (IR), though there is also a tiny amount of radio, and microwaves. Your eyes can't see light with such a long wavelength, so in a dark room, you would see me using an infra-red camera, but not a regular visible-light camera. Hotter things, such as filaments in light bulbs, do radiate light that can be seen. The surface of the Sun, at around 6000 Kelvin, also creates ultraviolet (UV) rays. And the blazing hot corona of the sun emits x-rays, and gamma rays, in addition to all of these previous kinds.
We normally talk about the different types of light as distinct objects. IR is "heat" radiation, because normally when we encounter it that's what it's doing: heating us. Then there's what most people call light, but which I call "visible light"; that kind of light is between 400 nm and 700 nm in wavelength, or between about 4.28 and 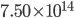 cycles per second. 1 cycle per second is called a Hertz (Hz). And if you have
cycles per second. 1 cycle per second is called a Hertz (Hz). And if you have 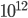 of them, that's a terahertz (THz). So visible light is 428 to 750 THz. Your eye was adapted to collect light of this type, so that's what you see.
of them, that's a terahertz (THz). So visible light is 428 to 750 THz. Your eye was adapted to collect light of this type, so that's what you see.
There are two things to consider. 1. What kinds of light does an object at temperature  radiate? and 2. How much light does it radiate? Radiation is constantly providing cooling and warming of objects, so we need to know the answers.
radiate? and 2. How much light does it radiate? Radiation is constantly providing cooling and warming of objects, so we need to know the answers.
A substance is always made up of atoms, and the atoms all have electrons. These electrons orbit the nucleus, but only at certain energies. These energies form discrete steps. When the atoms knock into each other, which they are constantly doing, there's a chance that the electron gets promoted to a higher step. If it picks up a lot of thermal energy from the atoms, it can suddenly jump up a few steps. After a short time, it will fall back down. If it falls down, a particle of light is generated, called a photon. Since the electron loses energy during that fall, the photon must be carrying away energy.
That's right, photons, which are what light is made of, have energy. The energy of a photon depends on its frequency or wavelength. If its frequency is 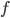 then its energy is
then its energy is 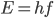 .
. 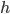 is just a constant, named for Max Planck, and called Planck's constant. If you'd prefer wavelength, it's
is just a constant, named for Max Planck, and called Planck's constant. If you'd prefer wavelength, it's 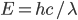 , where
, where 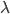 is the wavelength and
is the wavelength and  is the speed of light. Light is characterized only by frequency (or wavelength). The different types of light (radio, microwave, IR, visible, UV, x-ray, and gamma ray) are determined solely by
is the speed of light. Light is characterized only by frequency (or wavelength). The different types of light (radio, microwave, IR, visible, UV, x-ray, and gamma ray) are determined solely by 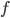 , and so by energy. Thus, the type of light emitted will have something to do with energy the electrons pick up, and hence on the temperature. On the other hand, the brightness only depends on how many photons there are. Brighter light, more photons. So what we have to figure out is how many photons of each type are made.
, and so by energy. Thus, the type of light emitted will have something to do with energy the electrons pick up, and hence on the temperature. On the other hand, the brightness only depends on how many photons there are. Brighter light, more photons. So what we have to figure out is how many photons of each type are made.
Here I'm going to write the amount of radiation emitted per second (the power) at each frequency for a body at temperature  . The only way I know to derive it is very advanced (a 4th year undergraduate physics major problem), so I'm not going to. After I write it, we can see its general features, and then we can try to figure out the total power radiated. Here it is:
. The only way I know to derive it is very advanced (a 4th year undergraduate physics major problem), so I'm not going to. After I write it, we can see its general features, and then we can try to figure out the total power radiated. Here it is:
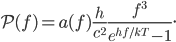
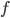 and
and  to this*. If you want to know the total energy being radiated, you just sum up the energies for all the possible frequencies, which we'll do next time. Here
to this*. If you want to know the total energy being radiated, you just sum up the energies for all the possible frequencies, which we'll do next time. Here 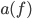 is called the emissivity, and it depends upon the object itself. If an object is perfectly absorbing, and reflects nothing, then
is called the emissivity, and it depends upon the object itself. If an object is perfectly absorbing, and reflects nothing, then 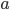 will be 1 for all frequencies. Otherwise,
will be 1 for all frequencies. Otherwise, 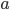 is somewhere between 0 and 1, and it might depend on frequency.
is somewhere between 0 and 1, and it might depend on frequency.
(*The above is actually per unit area. The bigger the object, the bigger the surface area, and the more photons. Also, since this is a distribution, we have to take an area to get an intensity, but this is a mathematical kink that the reader should not be worried about)
Here's what the spectrum looks like for an object at 300K:
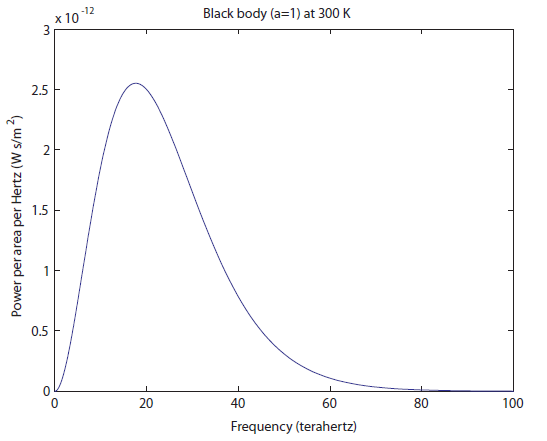 For simplicity we'll say this is a black object like coal. The area under the curve represents the total brightness, or the number of photons coming off. The range on the
For simplicity we'll say this is a black object like coal. The area under the curve represents the total brightness, or the number of photons coming off. The range on the 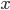 -axis is all in the "far infrared" segment of the electromagnetic spectrum. All of the frequencies lower than this are squished into the far left of the graph, so they aren't visible. Here's a close-up of the left part of the graph:
-axis is all in the "far infrared" segment of the electromagnetic spectrum. All of the frequencies lower than this are squished into the far left of the graph, so they aren't visible. Here's a close-up of the left part of the graph:
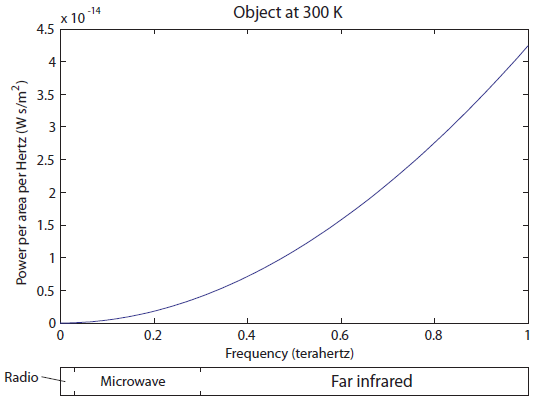 There is very little radio being emitted, a tiny amount of microwaves, and then we get into the Far IR. It's very tempting to just say that objects at room temperature only radiate IR. The fraction of light emitted in the radio/microwave range is only 0.0005%, which I determined by comparing the area under the curve for the radio and microwave parts to the area under the curve for the rest. It's not nothing, but it's quite small.
There is very little radio being emitted, a tiny amount of microwaves, and then we get into the Far IR. It's very tempting to just say that objects at room temperature only radiate IR. The fraction of light emitted in the radio/microwave range is only 0.0005%, which I determined by comparing the area under the curve for the radio and microwave parts to the area under the curve for the rest. It's not nothing, but it's quite small.
Now let's look at what happens for an object at twice the temperature, 600 K, which is hotter than your oven gets:
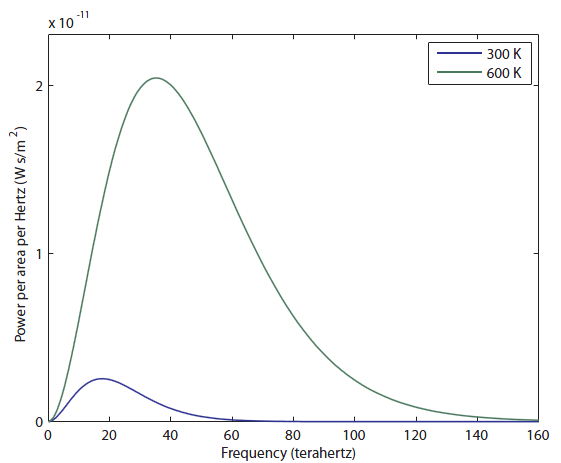 Wow, the 600 K object radiates a lot more light! I would say, just to eyeball it, that the area under the green curve is more than 20 times larger than the blue curve, and the area, as I've said, corresponds to more photons ("brighter", in a sense, except you can't actually see it). It also goes to larger frequencies than the 300 K object, but it's still very much in the infrared region. It has not even gotten to the "near infrared" region, which is above 300 THz (and which some insects can see). So we think that probably things have to be quite hot before they start to radiate light we can see. Notice that the peak frequency (the frequency that the most photons come off with) has also moved to the right, from about 18 to 40 THz. We'll think about that again later.
Wow, the 600 K object radiates a lot more light! I would say, just to eyeball it, that the area under the green curve is more than 20 times larger than the blue curve, and the area, as I've said, corresponds to more photons ("brighter", in a sense, except you can't actually see it). It also goes to larger frequencies than the 300 K object, but it's still very much in the infrared region. It has not even gotten to the "near infrared" region, which is above 300 THz (and which some insects can see). So we think that probably things have to be quite hot before they start to radiate light we can see. Notice that the peak frequency (the frequency that the most photons come off with) has also moved to the right, from about 18 to 40 THz. We'll think about that again later.
Now, let's increase the temperature until we see the object start to glow red. Here's the spectrum at 1600 K:
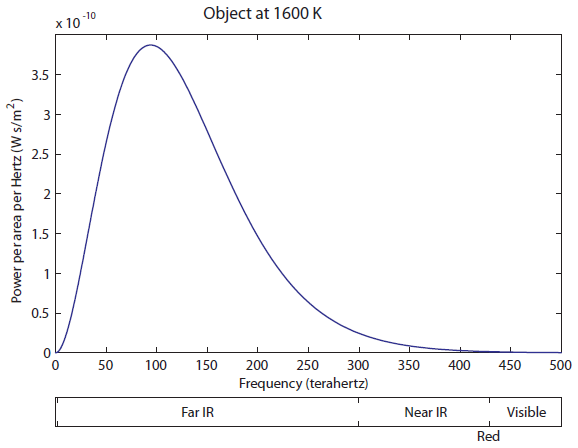 The tail on the right is finally barely overlapping the visible. A huge, huge majority of the light is still in the Far IR part. This thing is still mostly radiating heat, rather than useful visible light. But your eyes are fairly sensitive, so you can actually see an object start to glow red at 1600 K (I have seen it myself). Now let's heat up to 2100 K:
The tail on the right is finally barely overlapping the visible. A huge, huge majority of the light is still in the Far IR part. This thing is still mostly radiating heat, rather than useful visible light. But your eyes are fairly sensitive, so you can actually see an object start to glow red at 1600 K (I have seen it myself). Now let's heat up to 2100 K:
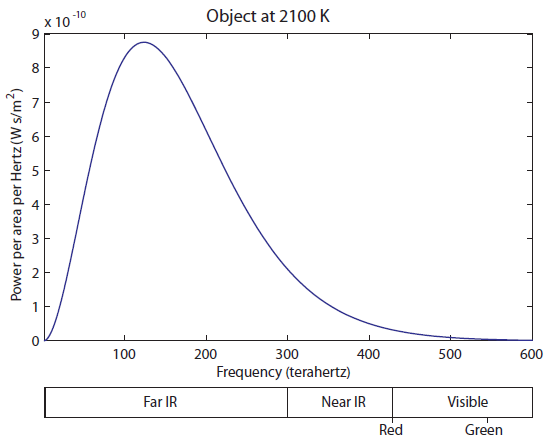 At 2100 K, you can see that there is significant overlap of the curve with red, and some overlapping with green. Red and green makes yellow, so as you increase the temperature, it turns red hot, and then yellow hot. It never turns "green hot", because the tail always has to start at infrared and go down. Any time green is emitted, red is also emitted. And, of course, once we turn the temperature up even higher, there will be blue photons, and we start to get white light, which is a combination of red, green, and blue.
At 2100 K, you can see that there is significant overlap of the curve with red, and some overlapping with green. Red and green makes yellow, so as you increase the temperature, it turns red hot, and then yellow hot. It never turns "green hot", because the tail always has to start at infrared and go down. Any time green is emitted, red is also emitted. And, of course, once we turn the temperature up even higher, there will be blue photons, and we start to get white light, which is a combination of red, green, and blue.
Now let's check out the surface of the Sun, which is about 6000 K:
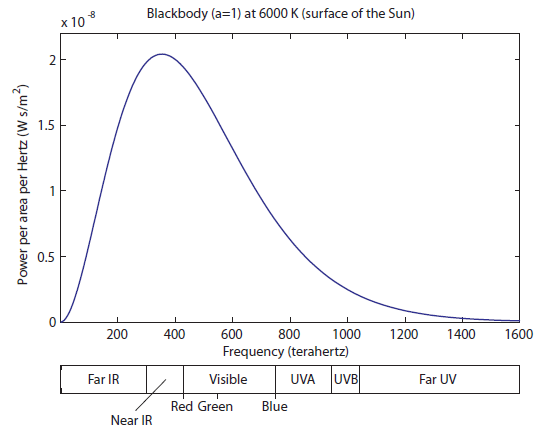 Here we still have a huge amount of IR radiation. A little less than half of the light coming off is still "heat" radiation, which can warm us up but can't be seen. The visible region is well represented, which means we have a significant amount of white light that is just a little bit less blue than it is red and green. Finally, we start to have significant amounts of radiation in the UV range. This is both the near-UV (which include UVA and UVB photons), and some far UV. Above UV would be X-rays and gamma rays. The surface of the sun does not create these; instead, they originate in the upper layer of hot gas around the sun called the Corona, for which the physics may be a bit different.
Here we still have a huge amount of IR radiation. A little less than half of the light coming off is still "heat" radiation, which can warm us up but can't be seen. The visible region is well represented, which means we have a significant amount of white light that is just a little bit less blue than it is red and green. Finally, we start to have significant amounts of radiation in the UV range. This is both the near-UV (which include UVA and UVB photons), and some far UV. Above UV would be X-rays and gamma rays. The surface of the sun does not create these; instead, they originate in the upper layer of hot gas around the sun called the Corona, for which the physics may be a bit different.
The phenomenon of objects radiating visible light when they get very hot is called incandescence. Regular old light bulbs use incandescence to create light. Here's what the electromagnetic spectrum for a tungsten filament in a light bulb ( equal to about 3000 K) looks like:
equal to about 3000 K) looks like:
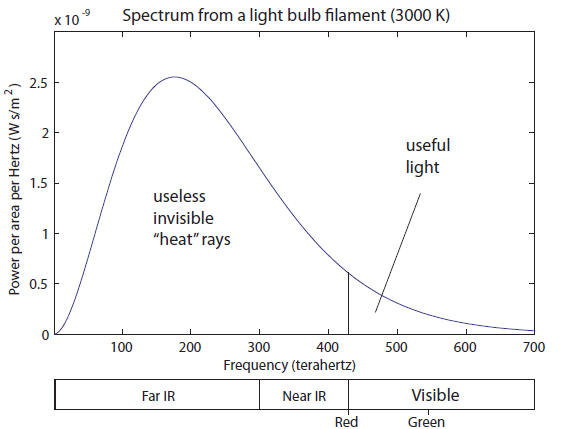 That sure seems like a dumb way to make light. Only about 20% of the photons are useful for seeing. The rest are just being radiated out as heat, warming up the glass bulb, the fixture, and your house. It is for this reason that it makes sense to ban incandescent light bulbs. The government has not yet seen fit to do so, but it should. We have much smarter ways of doing this.
That sure seems like a dumb way to make light. Only about 20% of the photons are useful for seeing. The rest are just being radiated out as heat, warming up the glass bulb, the fixture, and your house. It is for this reason that it makes sense to ban incandescent light bulbs. The government has not yet seen fit to do so, but it should. We have much smarter ways of doing this.
Next time we'll look more carefully at the total amount of radiation coming off something at temperature  , and we'll also start to investigate what happens when the object is not totally absorbing. We'll look at how things receive radiation, and so why temperature has to be given "in the shade" to be comparable from day to day. We'll see that we feel warmer when this radiation is incident upon us. And we'll see how reflection moderates how hot things get when sitting out in the Sun. Eventually, we'll be able to start talking about the phenomenon of climate change using this information.
, and we'll also start to investigate what happens when the object is not totally absorbing. We'll look at how things receive radiation, and so why temperature has to be given "in the shade" to be comparable from day to day. We'll see that we feel warmer when this radiation is incident upon us. And we'll see how reflection moderates how hot things get when sitting out in the Sun. Eventually, we'll be able to start talking about the phenomenon of climate change using this information.





