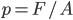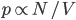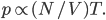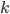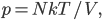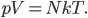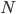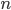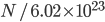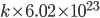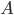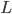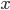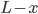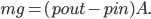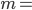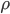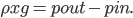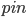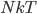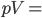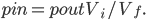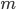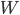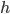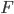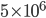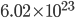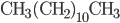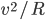Thermo for Normals is a short book (~100 pages) I wrote that conveys the basics of thermodynamics without all of the mathematical complexity. Over the next year I will be posting 1 or 2 sections of the text per week. I don't know whether I will ever try to publish it in any other form, though perhaps as an Amazon e-book it wouldn't be a bad idea. Questions, comments, criticisms, and corrections are welcome.
Evaporation and boiling
Drying puddles
If water boils at 212°F, which is when the liquid to gas transition happens, then how is it that a puddle can ever dry up? Surely the temperature is nowhere near boiling, yet large puddles can evaporate over a matter of hours.
The reason is that the molecules in the liquid aren't all going the same speed. Some of them are going faster and some are going slower. Some of the ones going faster have enough oomph to jump right out of the liquid if they're near the surface. After this happens, the liquid has a slightly lower temperature. This is because the temperature corresponds to the average energy per particle. And if the fastest particles leave, surely the average goes down. So the puddle is a little cooler, but it can absorb heat from the ground to put it back at room temperature. Then the process repeats.
Boiling the water, however, is way a much faster way of evaporating it. One you reach 212°F, any more heat added will go directly into particles escaping the fluid.
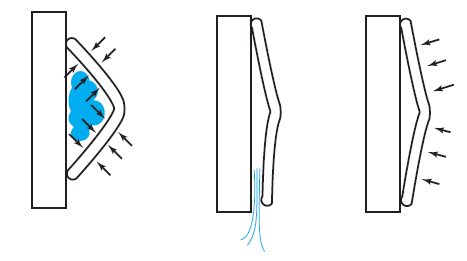
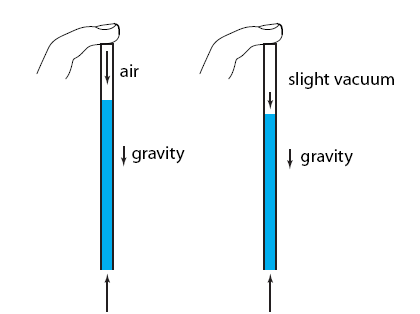
But what exactly is it that these particles have to overcome? Why do some of the particles need enough oomph to get out? What's stopping them? When the water is sitting in the atmosphere, it's constantly being bombarded by air molecules. So, at 212°F you can think that many particles have enough energy to overcome the pressure that the air is exerting, and at room temperature relatively few of them have that much energy. This poses an interesting question: if you reduce the air pressure, is it easier for the water to boil? Yes! In Denver, where the air is "lighter" (actually lower pressure), the boiling point is around 10°F lower than at sea level. This also means that the highest temperature that liquid water can be in Colorado is 202°F, to the consternation of tea drinkers, who insist that the water has to be warmer than that to brew a good cup. They could fix this by finding a way to add pressure to the gas above the water, which is what a pressure cooker does. In fact, in a pressure cooker the temperature of the liquid can be almost as hot as you want it to be. But I doubt we'll be seeing expatriate Brits brewing tea in a pressure cooker anytime soon.
By the way, there's another cool effect that can happen when going to Denver. Bags of chips are packaged at around sea level, which means that inside the sealed bag there is 14.7 psi of air. When being transported to Denver, where the pressure is significantly lower, the air inside the bag will push harder than the air outside the bag, which makes the bags inflate to their limit. Bags are reported to pop open spontaneously when being transported. My guess is that the chip makers have strengthened their bags to prevent this. Over time, tiny leaks in the bags make the pressure equilibrate so that they go back down to normal inflation. So if you're looking for fresh chips in Denver, check for the bags that look ready to pop, because they still have the sea-level pressure of gas in them.
Dying in space
How long can you live in outer space without a space suit? We've seen this in movies before. In the (terrible) movie Mission to Mars, for instance, the character of Woody takes off his helmet while in space and instantly freezes. And this is pretty close to what would actually happen. The absence of any air would make the water molecules in your body have little barrier to jumping out of your skin. All of the fast molecules will rapidly leave, taking away with them most of your thermal energy and causing a sudden catastrophic reduction in temperature.
Superheating
It is actually possible, though difficult, to heat liquid water above its boiling point even without applying pressure. The easiest way to do this is with a microwave and a glass bottle that has a relatively small neck. The molecules down in the fluid would love nothing more than to move to the surface and jump out. To do this they have to combine with other vaporized particles to form the bubble. But the bubble itself is a little hard for them to form; it takes a bit of energy to overcome the surface tension. In my high school chemistry class, they gave us a little rock to put into the water to help this start happening. It's because near the surface of the rock, the energy needed to form the bubble is reduced. But more often, the energy to form the bubble is provided by a mechanical shock, such as stirring the fluid.
If you can keep the fluid very still, the vapor molecules will be unable to form large enough bubbles to percolate out, and the rolling boil you're used to won't occur. But give the bottle just a little tap and the water will instantly flash boil!
Condensation and vaporization
When you take a hot shower, the room gets steamy (unless it's well ventilated). This does not happen with a cold shower, or even with a not-so-hot shower. With the description I gave earlier, it's fairly easy to understand why. Higher temperature means more water molecules can jump out of the liquid into the vapor phase. And then the mirror gets "steamy", which is water vapor condensing back into liquid, as tiny droplets, on it. This happens because the mirror is relatively cool compared to the air in the room. Water also condenses on the cool walls and ceiling, but it isn't as easy to see.
If you want to clear the mirror, you could wipe it down, or you could use a hair dryer. The hair dryer warms the water that has condensed up and so it re-evaporates (this tends to happen quickly, since hair dryers get ungodly hot).
The same thing happens inside a car. Humans secrete a fair amount of water vapor by breathing, and it tends to accumulate on the nearest cool surface, like the windshield. Now, here's something interesting. When you run the defroster, it doesn't seem to matter whether you use hot air or cold air; it will clear the condensation either way. How can cool air get rid of condensation?
Typical car ventilation systems allow for hot, cold, recirculating, or fresh air, with or without the AC on. You first might notice that the defroster does not work when using recirculating air with no AC unless the air is quite hot. All you're doing is moving the air around. But when the AC function is on, or when fresh air is coming in, the air passing over the glass is drier than the air in the car. What happened with the AC off is that the condensed liquid came to equilibrium with the water vapor in the air.
This is the same condition that hot coffee reaches within seconds. Water molecules jump into vapor until there are as many molecules jumping out as jumping back in. If you blow on the coffee, you remove the equilibrium and allow more molecules to jump out.
So when you blow air conditioned air over the windshield, you upset the equilibrium and cause water to jump out of the liquid to restore it. This will work with either warm or cold air.