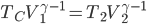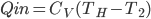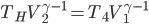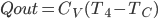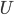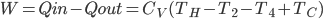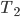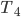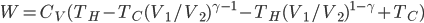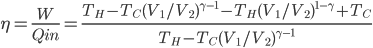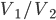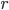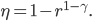There are lots of processes in thermo that we can think of that are irreversible. That means that if you ran a film of the process backwards, what you see would never happen. When you put an ice cube into lukewarm tea, the ice cube melts. If you run the film backwards, you see a cold drink suddenly warm up while simultaneously forming an ice cube. This doesn't violate energy conservation, but it never happens.
Or consider mixing two gases of different types. If you have nitrogen in one bottle, and oxygen in another, and you hook them together, the gases both fill the total available space and mix. But if you run the film backwards, you see a mixture of gas spontaneously segregate: all the nitrogen rushes to one and all the oxygen to the other. This never happens. You could wait billions of years and never see it.
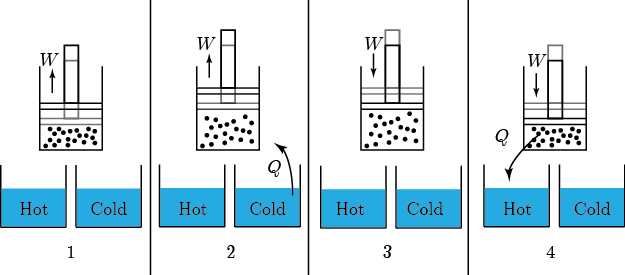
Earlier we discussed The Carnot engine, which had 4 stages (called strokes, if you're interested):
- expansion at
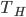 doing work
doing work - expansion where temp changed from
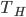 to
to 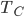 .
. - compression at
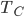
- compression from
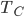 to
to 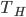
A normal car engine has the same number of stages
- Air and gasoline are let into the piston and compressed.
- The mixture is ignited, rapidly increasing the temperature.
- The piston extends, doing work.
- The combusted gases are expelled
When the gasoline/air is ignited in step 2, the hydrocarbons undergo combustion, making carbon dioxide and water vapor, plus a bunch of heat.
Now, here's a funny question: Could either of these two engines can be run backwards?
For the Carnot engine the answer is: absolutely. It would work the following way. The most important thing to observe (which may not be totally obvious) is that the net effect is to take in work from somebody and move heat from the cold bath to the hot bath.
- Start with the gas at
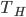 and allow the piston to push out until the temp falls to
and allow the piston to push out until the temp falls to 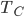 . No heat is absorbed.
. No heat is absorbed. - Continue allowing it to expand in contact with the cold bath. It will absorb heat to keep its temp from dropping.
- Now push it in while not letting heat in or out, which will increase its temperature to
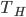 .
. - Push while it's in contact with the hot bath, so that it gives heat to the bath to remain at
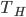 .
.
The net effect is to take in work and move heat from the cold bath to the hot one, which is what a refrigerator does. For this reason, this is called a Carnot refrigerator. It's the same as the diagram for the Carnot engine, but with all the directions reversed.
But the car engine can most definitely not be reversed. Think of it: you shove the piston down and the CO2 and water produced in combustion spontaneously turn back into gasoline and air ... no way could that happen!
We also know that the Carnot engine is very efficient, whereas the car engine is not. This is not a coincidence. It is almost as if there is a "right way" and a "wrong way" to do things. Slow and reversible is right, fast and irreversible is wrong. (In no way should these "right" and "wrong" designations be construed as normative judgements.)
It is, in fact, possible to prove that the Carnot is better than any other engine between the same two temperatures. But since car engines are the most likely kind of engine you will ever see, let's just analyze that one.
The description of the four stages above is accurate, but we'll have to change the way we phrase things to determine the efficiency as compared to a Carnot engine. The cycle, called the Otto cycle (that shouldn't be too hard to remember!) is in every important way the same as this:
- Some gas in a piston is compressed from
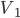 to
to 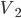 with no heat from the outside.
with no heat from the outside. - The gas is held at constant volume while it is put into contact with a heat bath at temperature
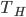 until its temp is
until its temp is 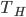
- The piston expands, doing work, with no heat from the outside.
- The gas is put into contact with a cold bath at
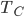 and allowed to cool to
and allowed to cool to 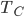 .
.
So, we get around the combustion and exhaust by substituting our hot bath and cold bath. In part 1 we do work, in 2 heat is absorbed, in 3 the engine does work, and in 4 heat is expelled. We can now calculate the efficiency
The efficiency of the Otto cycle (last equation in the aside above) does not depend on the two temperatures, but rather on the compression ratio, or the volume of the piston when it's expanded divided by the volume when it's compressed. In modern engines this can be as high as 14 or so. Nevertheless, even for a very large compression ratio the efficiency is lower than the Carnot engine operating between the same two temperatures. Note that this is for an ideal Otto cycle, without friction in the piston etc., so car engines will never realize this.
Considering the temperatures most often used, with the hot temperature at about 1000 K and the cold temperature at about 300 K, you see the efficiency vs. compression ratio for the two engines displayed below.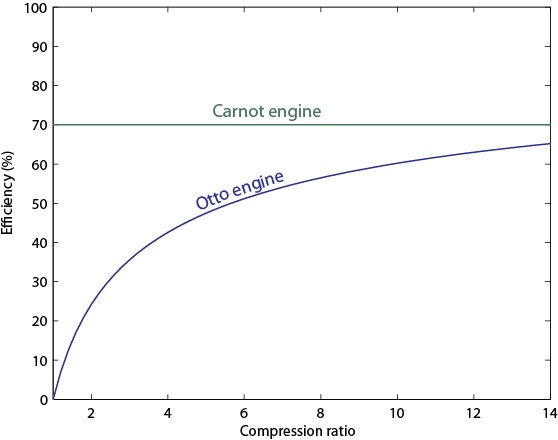
(Of course, you can make a Carnot engine with low efficiency by operating it at two temperatures that aren't very different. I'm only addressing practical temperatures here. It is not the case that every Otto engine is worse than every Carnot engine, but it is the case that with only two heat reservoirs available, the Carnot is more efficient.)
But why is the Carnot always better? Why is reversibility always the best for efficiency? We must be doing something fundamentally different. But what could it be?